- Newsroom
- Research Progress
- Academic Events
- Join Us
Furthermore, we took lysozyme as the basic model protein to investigate the kinetics, driving forces on protein loading and factors controlling loading efficiency into porous Hep/CaCO3 particles (Fig. 2). As revealed, the adsorption process obeyed the pseudo second-order kinetics and Langmuir adsorption model. Doping heparin greatly influenced the detailed texture, pore size, surface area, and maximum loading capacity of lysozyme. Accompanying with pH change, the lysozyme orientation shifted from “side on” at lower pH to “end on” at pH around IEP. At proper concentration of NaCl (CNaCl), the loaded lysozyme could be released from Hep/CaCO3 particles, making them available for lysozyme reloading. Most importantly, such release-reloading cycle didn’t disturb the bioactivity of released lysozyme and following reloading ability. This work was published in Colloids and Surfaces B: Biointerfaces 2019, 175, 184-194.
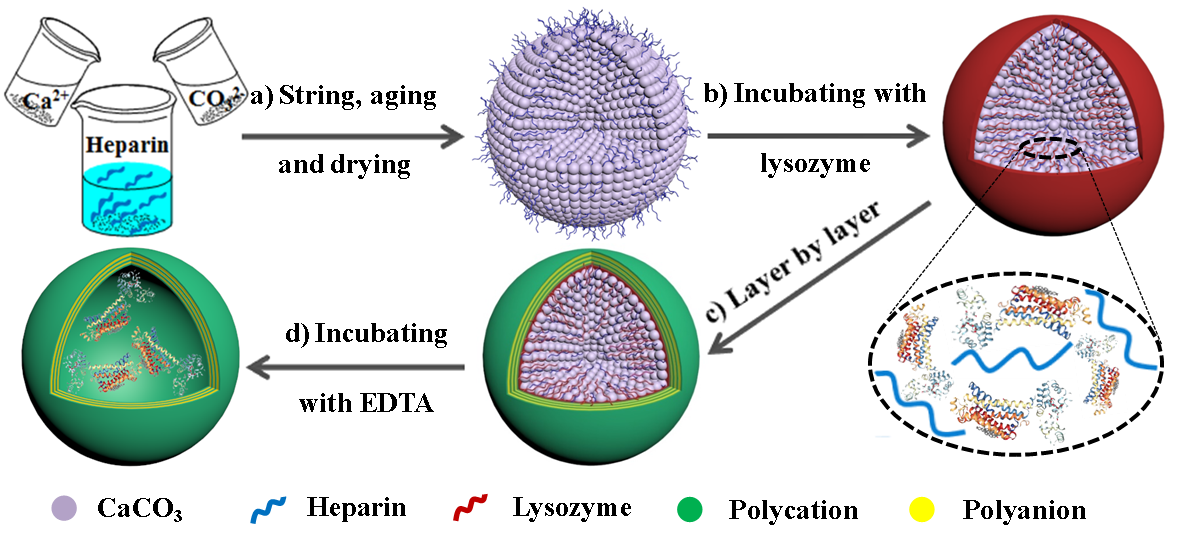
Fig. 1. Schematic illustration of (a) Hep/CaCO3 particle synthesis, (b) protein loading, (c) protective layers construction, (d) sacrificing the template to generate the polyelectrolyte capsules with encapsulated protein.
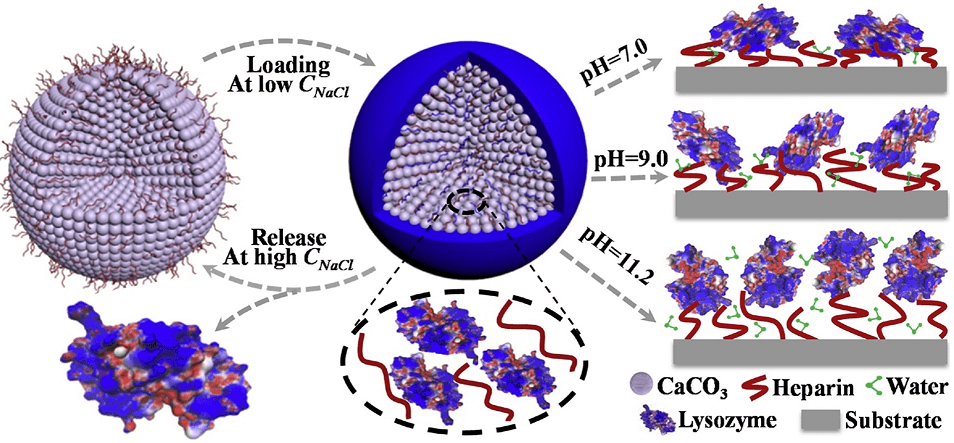
Fig. 2. Schematic illustration of driving forces on protein loading and factors on protein loading.